
CO Lewis Structure ,Valence Electrons ,Formal Charge ,Polar or Nonpolar
How to Draw the Lewis Dot Diagram for Carbon monoxide (CO) Wayne Breslyn 726K subscribers Join Subscribe Subscribed 774 108K views 3 years ago A step-by-step explanation of how to draw the CO.
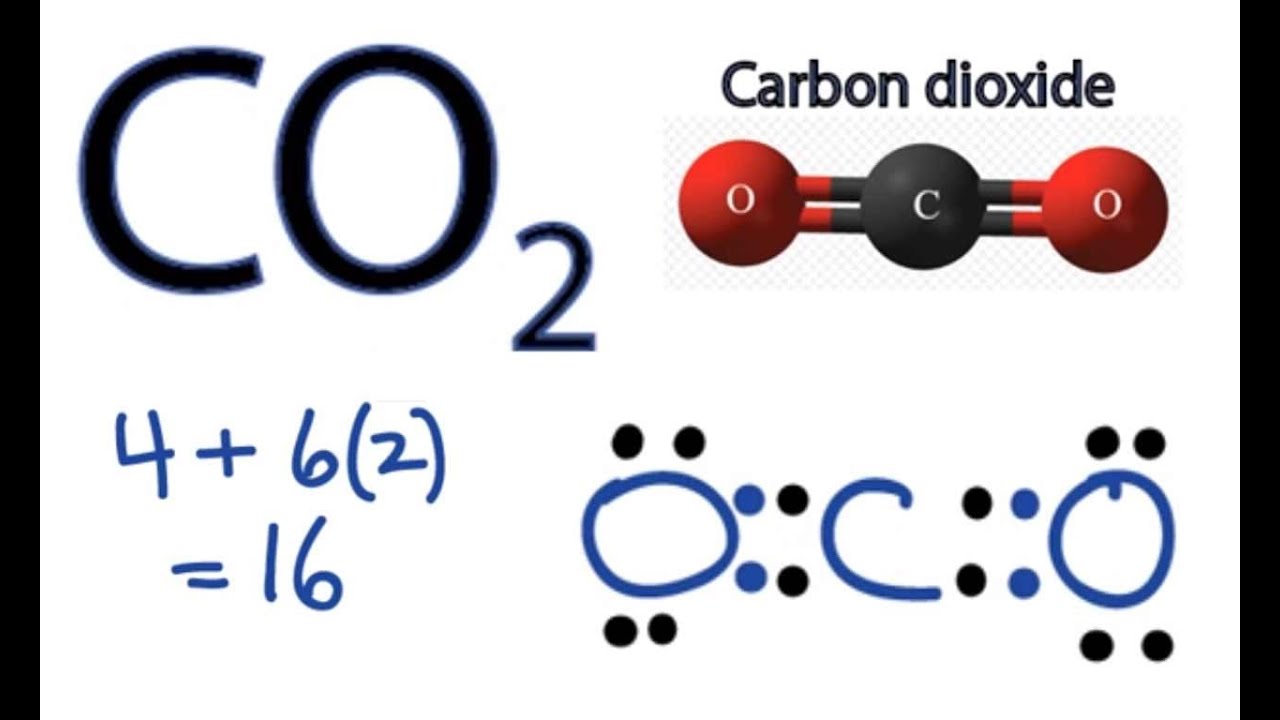
CO2 Lewis Structure How to Draw the Dot Structure for Carbon Dioxide
By using the lewis concept we understand the bonding between atoms in a carbon and oxygen molecule. So the hybridization of carbon and oxygen in CO are sp. This is a linear molecule geometry. The formal charge of carbon monoxide is zero. We can count charge of individual also. How to draw Lewis structure for CO ?
Co Lewis Structure Molecular Geometry
Lewis: CO is a two electron donor. Transition metals are electrophiles. CO binds to metal atoms or ions. The carbon is the usual donor atom; it has a lone pair and a negative formal charge. The donation of an electron pair to a metal cation is shown in figure 4.2. 2. Figure 4.2. 2: Binding of CO to a metal cation.

Write the Lewis dot structure of CO molecule.
The formula for calculating the formal charge (FC) is: FC = Valence electrons - Non-bonding electrons - 1/2 * Bonding electrons. For carbon in CO, the valence electron count is 4, and in the Lewis structure, it has two lone pairs and a double bond with oxygen. Therefore, FC of carbon = 4 - 2 - 1/2 * 4 = 0. For oxygen in CO, the valence.
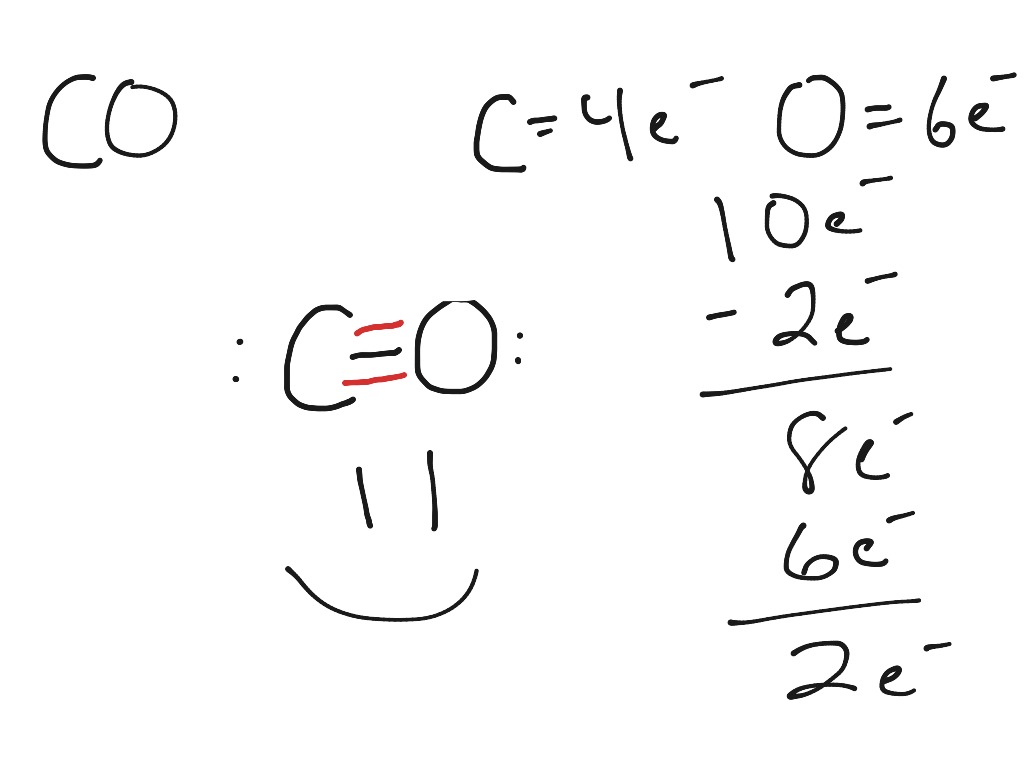
Electron Dot Diagram For Carbon General Wiring Diagram
Steps Sketch the structure Location of carbon and oxygen on the periodic table To start drawing the CO Lewis structure, the first step is to determine the total number of valence electrons in the molecule. The total valence electrons in the molecule can be calculated by multiplying the valence electrons of each atom.

CO Lewis Structure and Formal ChargeCarbon monoxide Lewis structure
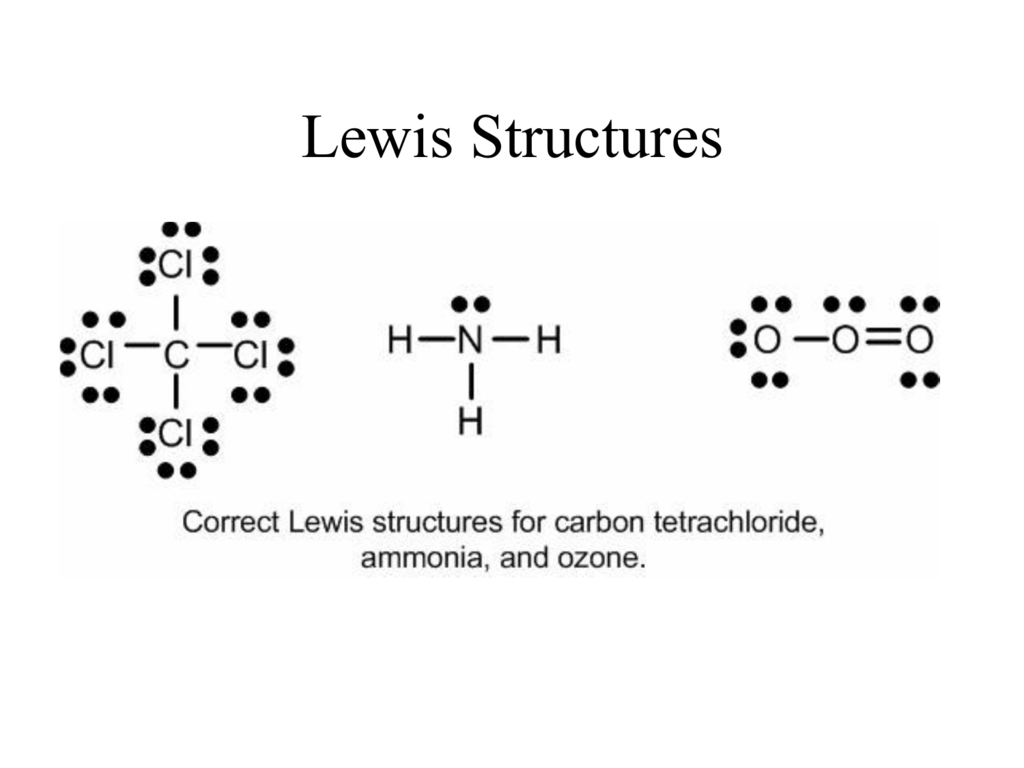
The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between the atoms). A dash (or line) is sometimes used to indicate a shared pair of electrons: A single shared pair of electrons is called a single bond.
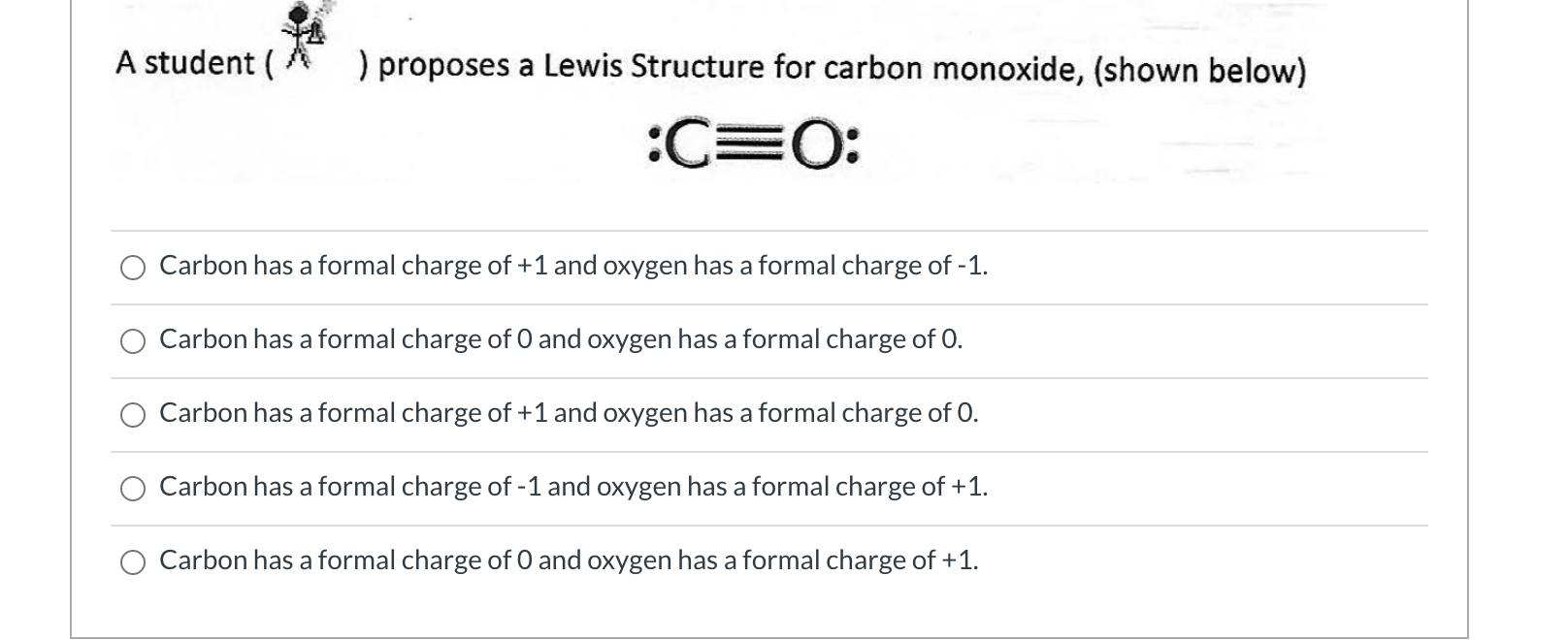
Solved A student ) proposes a Lewis Structure for carbon
Lewis diagrams © 2024 Khan Academy Terms of use Privacy Policy Cookie Notice Drawing Lewis diagrams Google Classroom About Transcript A Lewis diagram shows how the valence electrons are distributed around the atoms in a molecule.
Lewis Dot Diagram For N Wiring Diagram
How to Draw the Lewis Dot Diagram for Carbon monoxide (CO) Watch on Steps of drawing CO lewis structure Step 1: Find the total valence electrons in CO molecule In order to find the total valence electrons in CO (carbon monoxide) molecule, first of all you should know the valence electrons present in a single carbon atom as well as oxygen atom.

CO Lewis StructureThis post is about carbon monoxide lewis structure
Now, step 2 becomes: Step 2: Total electrons needed for 6 octets + one 12-tet = 60. Step 3: Total shared/bonding electrons = 60 - 48 = 12. Step 4: Figure 5: This example suggests that step 6 of the usual Lewis structure rules should be replaced by. 6'. Assign lone pairs to the terminal atoms to give them octets.
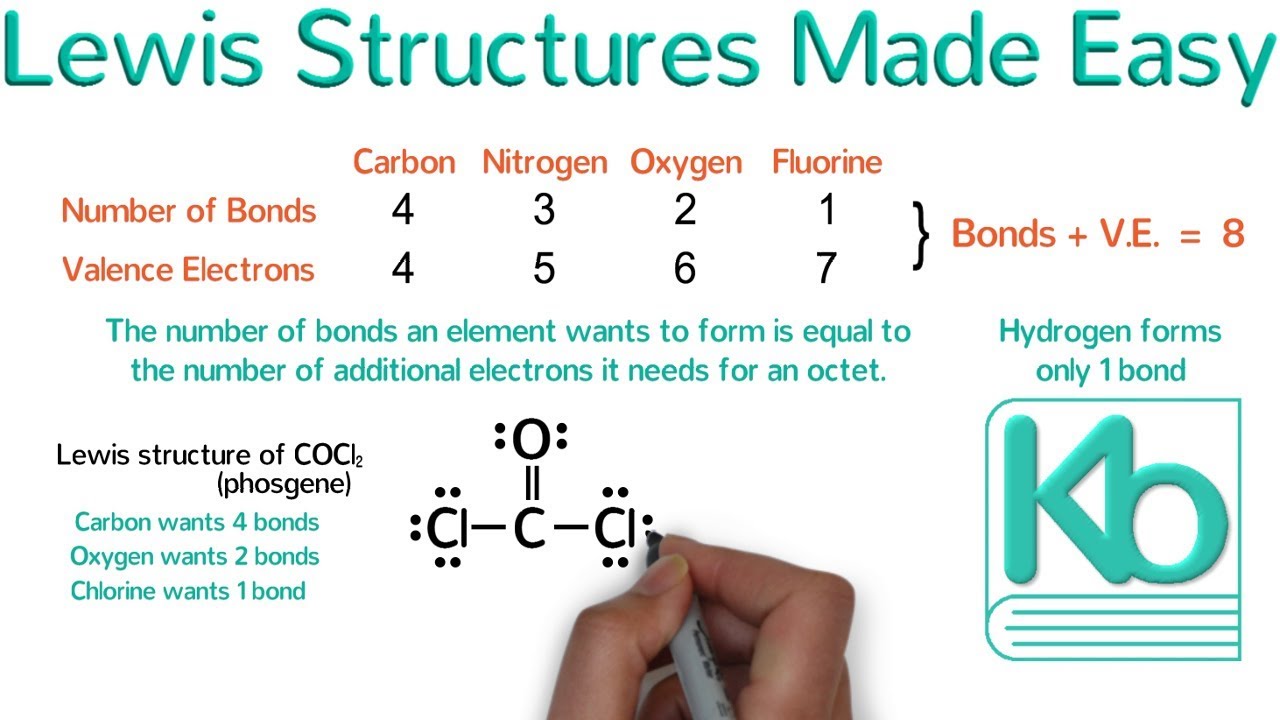
Lewis Structures Made Easy Examples and Tricks for Drawing Lewis Dot
Example: drawing the Lewis structure of CO 3 2 - Step 1) Figure out how many electrons the molecule must have. Carbon has 4 valence electrons. Each oxygen has 6 valence electrons. The -2 charge means that there are 2 extra electrons. Total: 4 + (3 × 6) + 2 = 24 electrons. The final answer MUST have this number of electrons‼!

CO32 Lewis Structure How to Draw the Lewis Structure for CO3 2
Draw the lewis diagram Geometrical Shape of the Carbon Monoxide (CO) The bond angle between the carbon and the oxygen atom is 180 degrees. It makes the geometrical structure of the carbon monoxide linear.
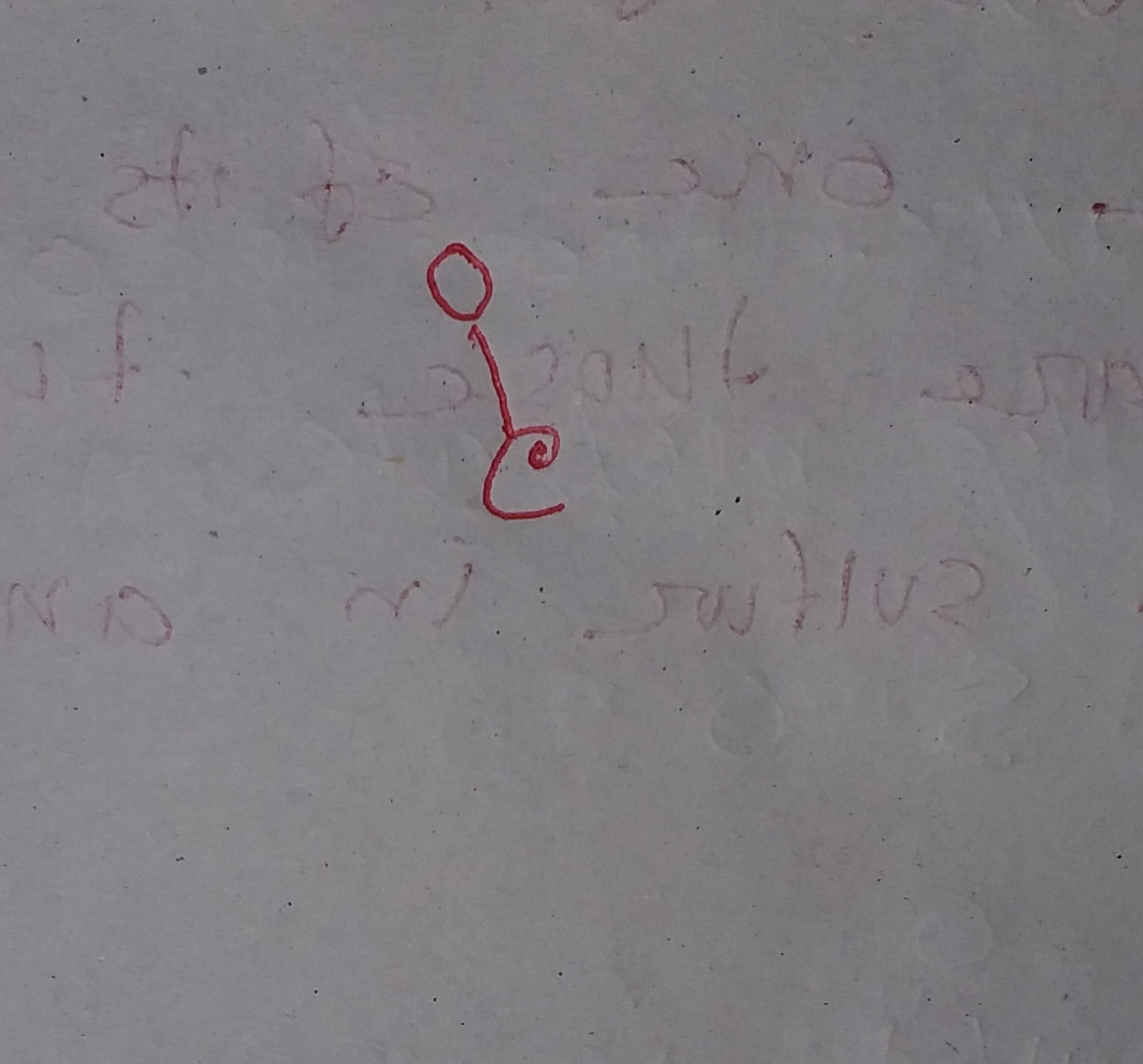
CO Lewis Structure ,Valence Electrons ,Formal Charge Carbon Monoxide
How to Draw the Lewis Dot Diagram for Carbon monoxide (CO) It is helpful if you: Try to draw the CO Lewis structure before watching the video. Watch the video and see if you missed any steps or information. Try structures similar to CO for more practice. List of Lewis Structures Lewis Structures for CO.
[Solved] 8. Draw the Lewis structures for CO2 and CO, and predict the
Lewis structures (also known as Lewis dot diagrams, electron dot diagrams,"Lewis Dot formula" Lewis dot structures, and electron dot structures) are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds. The Lewis structure.
/ScreenShot2018-11-19at11.40.52PM-5bf3909a46e0fb00510dbd6d.png)
Lewis Structure Definition and Example
C O Now keep the valence electrons of both Carbon and Oxygen atoms around it like this. You might notice Carbon has only four valence electrons and needs four more to complete its octet. In contrast, Oxygen has six valence electrons and only needs two more electrons to complete the octet.

CO Lewis Structure ,Valence Electrons ,Formal Charge ,Polar or Nonpolar
The N atom has the following Lewis electron dot diagram: It has three unpaired electrons, each of which can make a covalent bond by sharing electrons with an H atom. The electron dot diagram of NH 3 is as follows: Exercise 12.4.2 12.4. 2. Use a Lewis electron dot diagram to show the covalent bonding in PCl 3. Answer.
CO Lewis Structure How to Draw the Dot Structure for CO YouTube
Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron.